
Discover the secrets of the brain with the epilepsy research being done at the SJD Barcelona Children's Hospital.
The brain is a very complex organ that processes huge amounts of information. At times, the connections inside it do not function properly, causing what are known as epileptic seizures. With the informational materials available at the Science Forum, you can learn more about how the human brain works and about the latest steps we have taken in studying and treating epilepsy at the SJD Barcelona Children's Hospital.
SJD Research
At the SJD Barcelona Children's Hospital, we research diseases that impact young children. We opt for translational investigation methods, which aim to translate basic biomedical research from the lab into real-life patient applications, helping to build knowledge and find new solutions to create innovative treatments.
Our research teams work to learn more about the epilepsy that manifests in childhood (its origin, the biological mechanisms that trigger it, or its link to several other mutations) and also to improve diagnosis.
Pediatric epilepsy research projectsComparative study of neuroimaging testsThe goal of this study was to compare different types of neuroimaging studies to figure out which study was most precise when determining the region of the brain in which focal epileptic seizures occurred. This will help medical professionals to perform more targeted surgeries.
More informationSJD ResearchNeuroimaging testing study
So that we can perform more targeted surgeries, we have carried out a study to identify the most precise neuroimaging testing method for finding the part of the brain in which focal epileptic seizures occur.
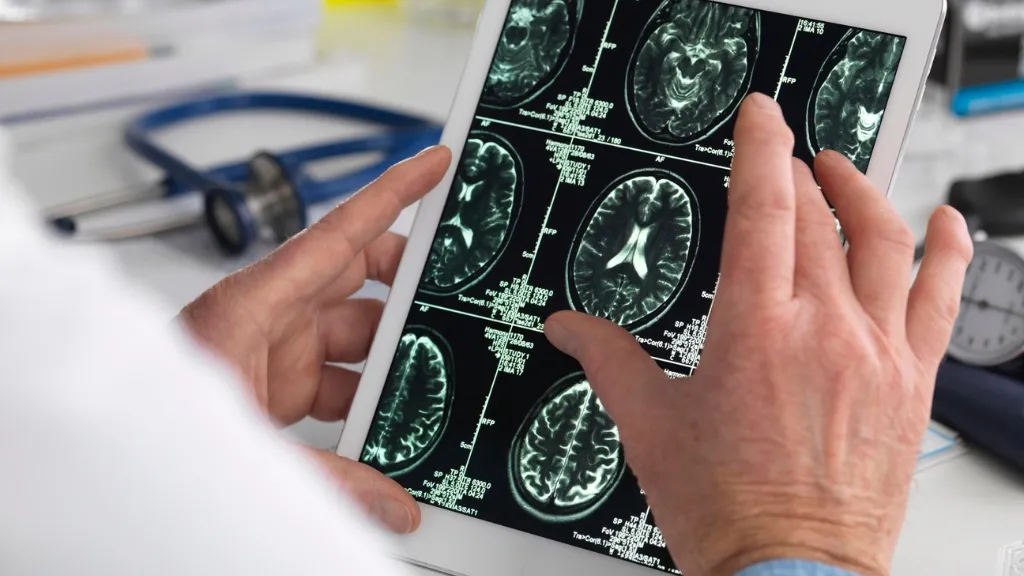
Surgery to treat epilepsy
At times, in cases where the epilepsy does not respond to pharmaceutical treatment, we recommend surgical intervention, as this achieves a significant reduction in seizures (more than 90%) in 2 out of 3 cases, sometimes even completely eliminating seizures. Early monitoring of the seizure is essential to avoid neurodevelopmental delays.
Comparative study of neuroimaging tests: Which test is most precise at identifying the region of the brain in which the epileptic seizure occurs?
We have assessed and compared the usefulness of PISCOM, a new neuroimaging technique, with SISCOM and 18F-FDG PET, two other imaging techniques used to evaluate epileptogenic zones. This is the part of the brain where epileptic crises originate. Removing this part of the brain can suppress seizures.
Look around the diagnostics and cerebral mapping room at the SJD Barcelona Children's Hospital:
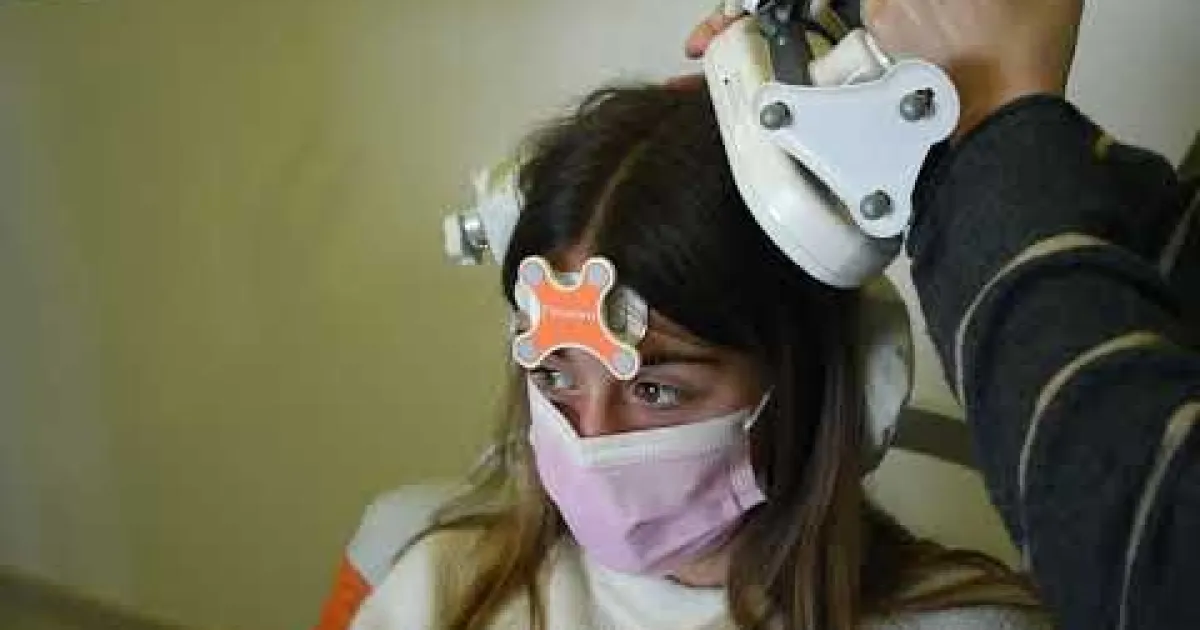 https://www.youtube.com/watch?v=1kQh_8DFpoM
https://www.youtube.com/watch?v=1kQh_8DFpoM GRINpathy studyWe have looked at the various effects certain GRIN gene mutations have on the NMDA receptor in paediatric patients (a glutamate receptor located in the neuronal membrane).
More informationSJD ResearchGRINpathy study
We have studied the effects of different gene mutations in children with GRINpathies (rare neurodevelopment disorders) on the NMDA receptor, a glutamate neurotransmitter receptor found in the neuronal membrane. The NMDA receptor functions abnormally in these patients due to mutations in GRIN genes.

NMDA glutamate receptors, proteins which are involved in neuronal synapses, can function abnormally because of mutations in GRIN genes, leading to disorders known as GRINpathies.
What is the synapse of a neuron? This refers to the space between two neurons, across which nerve impulses are transmitted. When there is an abnormality in the synapses, it becomes difficult for neurons to communicate with one another and with other cells in our body, causing neurological diseases and/or disorders.
GRINpathies cause the brain to develop improperly, which can lead to epileptic seizures. It is vital to understand the impact that GRIN gene mutations can have on NMDA glutamate receptors so that we can look for possible treatments.
Study on the functional impact of GRIN gene mutations, which cause NMDA glutamate receptor abnormalities
By using a 3D model of the NMDA receptor and other kinds of methods, the specific symptoms caused by various GRIN gene mutations have been able to be studied. Additionally, this 3D model also makes it possible to look into potential treatments.
Along these lines, the ‘patch-clamp’ technique was used for more in-depth study of changes to the NMDA receptor by various GRIN gene mutations. Let us explain with a video!
The ‘patch-clamp’ technique
 https://www.youtube.com/watch?v=z_dfBQeRTvw
https://www.youtube.com/watch?v=z_dfBQeRTvw KCNQ2-type channelopathy studyThe aim of this study was to find specific biomarkers for KCNQ2-type channelopathy, as well as study its symptomatology, among which is epilepsy. We also hoped to analyse how different treatments worked on mutated channels.
More informationSJD ResearchChannelopathy study
To better understand KCNQ2-type channelopathies, we investigated which specific symptoms are caused by the many mutations that can cause it (including epilepsy) and we have analysed the effect of treatments that act on dysfunctional ion channels. KCNQ2-type channelopathies are neurological diseases caused by several gene mutations in the KCNQ2 gene, which codes for a potassium ion channel found in neurons.
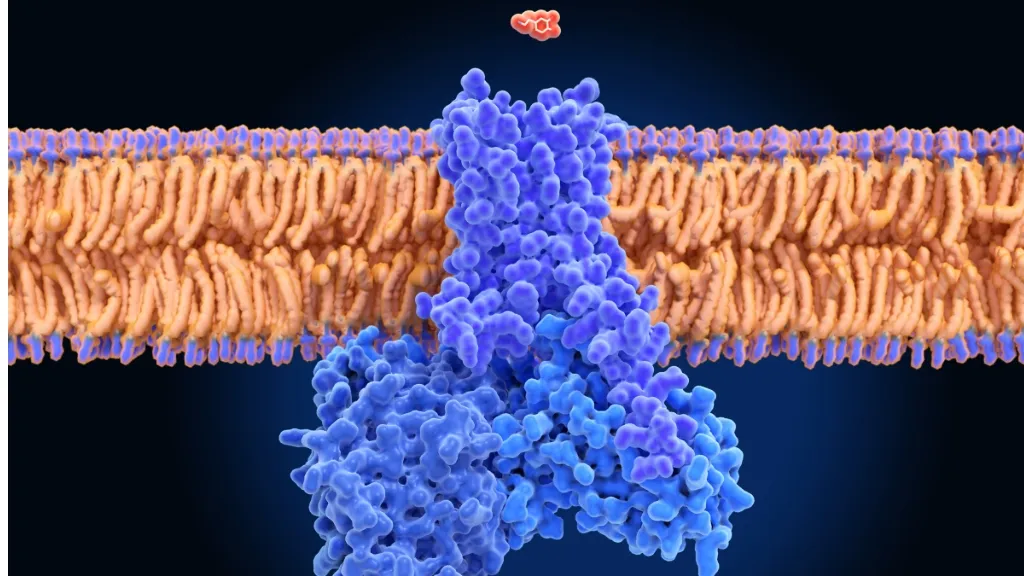
Mutations in neuronal membrane channels
The neuronal membrane has a series of proteins that act as channels, allowing for the circulation of ions. Ions are involved in the transmission of nerve impulses (a signal that goes from one neuron to another, sending information to cells in the body).
Some mutations cause an abnormality in these channels, hindering synapse function (the space between two neurons, across which nerve impulses are transmitted, allowing for them to communicate). These abnormalities, known as channelopathies can cause epilepsy and other neurological symptoms. It is essential to understand, on the one hand, how each mutation in genes that code for these channels affects the brains of newborns, and on the other hand, how epilepsy affects their neurodevelopment. It is also important to test which treatments work best in each specific case.
BIO-KCNQ2 Project KCNQ2 channelopathy study: analyses of biomarkers, mutation effects and treatment efficiency assessment.
We carried out genetic panels on a group of children to detect KCNQ2 gene mutations that cause epilepsy. We also carried out neurological tests and utilised medical assessment scales to describe symptoms and neurodevelopment issues brought on by each mutation. Finally, we administered several medications to patients to understand their effects in each different case.
The goal of this study was to compare different types of neuroimaging studies to figure out which study was most precise when determining the region of the brain in which focal epileptic seizures occurred. This will help medical professionals to perform more targeted surgeries.
Neuroimaging testing study
So that we can perform more targeted surgeries, we have carried out a study to identify the most precise neuroimaging testing method for finding the part of the brain in which focal epileptic seizures occur.

Surgery to treat epilepsy
At times, in cases where the epilepsy does not respond to pharmaceutical treatment, we recommend surgical intervention, as this achieves a significant reduction in seizures (more than 90%) in 2 out of 3 cases, sometimes even completely eliminating seizures. Early monitoring of the seizure is essential to avoid neurodevelopmental delays.
Comparative study of neuroimaging tests: Which test is most precise at identifying the region of the brain in which the epileptic seizure occurs?
We have assessed and compared the usefulness of PISCOM, a new neuroimaging technique, with SISCOM and 18F-FDG PET, two other imaging techniques used to evaluate epileptogenic zones. This is the part of the brain where epileptic crises originate. Removing this part of the brain can suppress seizures.
Look around the diagnostics and cerebral mapping room at the SJD Barcelona Children's Hospital:

We have looked at the various effects certain GRIN gene mutations have on the NMDA receptor in paediatric patients (a glutamate receptor located in the neuronal membrane).
GRINpathy study
We have studied the effects of different gene mutations in children with GRINpathies (rare neurodevelopment disorders) on the NMDA receptor, a glutamate neurotransmitter receptor found in the neuronal membrane. The NMDA receptor functions abnormally in these patients due to mutations in GRIN genes.

NMDA glutamate receptors, proteins which are involved in neuronal synapses, can function abnormally because of mutations in GRIN genes, leading to disorders known as GRINpathies.
What is the synapse of a neuron? This refers to the space between two neurons, across which nerve impulses are transmitted. When there is an abnormality in the synapses, it becomes difficult for neurons to communicate with one another and with other cells in our body, causing neurological diseases and/or disorders.
GRINpathies cause the brain to develop improperly, which can lead to epileptic seizures. It is vital to understand the impact that GRIN gene mutations can have on NMDA glutamate receptors so that we can look for possible treatments.
Study on the functional impact of GRIN gene mutations, which cause NMDA glutamate receptor abnormalities
By using a 3D model of the NMDA receptor and other kinds of methods, the specific symptoms caused by various GRIN gene mutations have been able to be studied. Additionally, this 3D model also makes it possible to look into potential treatments.
Along these lines, the ‘patch-clamp’ technique was used for more in-depth study of changes to the NMDA receptor by various GRIN gene mutations. Let us explain with a video!
The ‘patch-clamp’ technique

The aim of this study was to find specific biomarkers for KCNQ2-type channelopathy, as well as study its symptomatology, among which is epilepsy. We also hoped to analyse how different treatments worked on mutated channels.
Channelopathy study
To better understand KCNQ2-type channelopathies, we investigated which specific symptoms are caused by the many mutations that can cause it (including epilepsy) and we have analysed the effect of treatments that act on dysfunctional ion channels. KCNQ2-type channelopathies are neurological diseases caused by several gene mutations in the KCNQ2 gene, which codes for a potassium ion channel found in neurons.

Mutations in neuronal membrane channels
The neuronal membrane has a series of proteins that act as channels, allowing for the circulation of ions. Ions are involved in the transmission of nerve impulses (a signal that goes from one neuron to another, sending information to cells in the body).
Some mutations cause an abnormality in these channels, hindering synapse function (the space between two neurons, across which nerve impulses are transmitted, allowing for them to communicate). These abnormalities, known as channelopathies can cause epilepsy and other neurological symptoms. It is essential to understand, on the one hand, how each mutation in genes that code for these channels affects the brains of newborns, and on the other hand, how epilepsy affects their neurodevelopment. It is also important to test which treatments work best in each specific case.
BIO-KCNQ2 Project KCNQ2 channelopathy study: analyses of biomarkers, mutation effects and treatment efficiency assessment.
We carried out genetic panels on a group of children to detect KCNQ2 gene mutations that cause epilepsy. We also carried out neurological tests and utilised medical assessment scales to describe symptoms and neurodevelopment issues brought on by each mutation. Finally, we administered several medications to patients to understand their effects in each different case.









